The latest issue of Nature highlighted four papers that unveiled new knowledge regarding the structure of glucagon-like peptide 1 (GLP-1) receptor, a B class or secretin family G-protein coupled receptor (GPCR). The structural studies capture the receptor in various states (active/inactive) bound to peptide agonists, allosteric modulators or G proteins.
GPCRs make up an important drug target class for a variety of reasons. A substantial proportion of the genome codes for GPCRs (~4%) and 25-30% of currently marketed drugs target GPCRs. The top selling GPCR targeting drugs reap in $5-9 billion / year/ drug, Furthermore, there are about 120 GPCRs where endogenous ligands are unknown (called orphan GPCRs), indicating much room for development potential. The well-established role that GPCRs play in signalling pathways in a variety of physiological functions and their involvement in disease, further supports their study as drug targets.
The structure of GPCRs however are notoriously difficult to study because they become highly unstable when taken out of cell membranes. This therefore makes it difficult to design potent drugs against them. The authors of one of the papers from UK-based Heptares Therapeutics counter this with their StaR® (stabilised receptor) technology. This involves introduction of point mutations which improve GPCR thermostability without affecting their pharmacology. Other authors go about it with other techniques such as cryo-electron microscopy (cryo-EM).
GPCRs have extracellular N-terminals, seven canonical transmembrane domains, and an intracellular C-terminal that regulates downstream signaling. This rather detailed diagram shows which parts are involved with what.
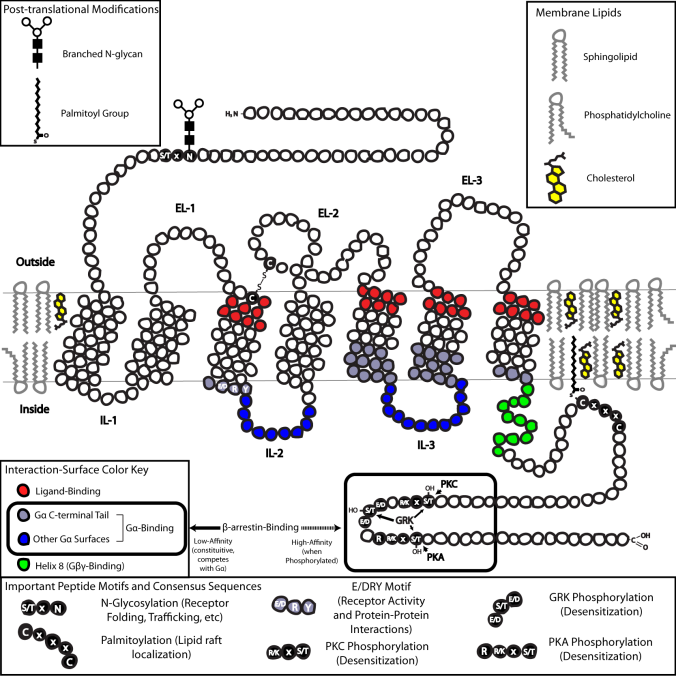
Image from Wikipedia Commons, read more about how GPCRs work here.
GLP-1 is a 30 amino acid long hormone, produced by intestinal cells and certain neurons in the brainstem. It controls blood sugar levels by binding to GLP-1 receptor in pancreatic beta cells to stimulate insulin secretion. The GLP-1 receptor is also expressed in the brain, where GLP-1 mediates appetite suppression and now recently, nicotine avoidance. Interestingly, GLP-1 also produced protective effects in neurodegenerative disease models and a clinical trial of Exendin-4 for Alzheimer’s disease was recently completed, with results yet to be released.
Sidenote: Exendin-4 is a hormone isolated from the Gila Monster (venomous lizard found in the US and Mexico) that closely resembles GLP-1, inducing the same glucose-regulating effects. A synthetic version is now marketed as Exenatide by Astrazeneca for the treatment of diabetes.
The new structural information unveiled allowed researches from Heptares Therapeutics to design more potent peptide agonists than the currently available Exendin-4, which showed efficacy in a mouse diabetes model. The authors modeled a peptide (peptide 5) to fit deep in the binding pocket of GLP-1 receptor with its C-terminus extending towards the extracellular portion of the receptor. They weren’t able to get the full-length structure of the peptide bound to the GLP-1 receptor, but they superimposed the known structure of GLP-1 peptide bound to only the extracellular domain (ECD) of GLP-1 receptor onto peptide 5, and found some differences. This they claimed to be due to the flexibility of the ECD or its altered behavior when expressed alone.
To improve the pharmacokinetics and stability of peptide 5, they introduced chemical modifications to the N-terminal end of the peptide producing peptide 6 and 7. Another peptide 8 was made by adding a PEG (polyethylene glycol) group which was predicted to extend its half-life by reducing proteolysis and increasing stability in plasma.
The latter worked best as peptide 8 showed the highest efficacy compared to other peptides in stimulating insulin secretion from isolated rat pancreatic islets. In mice that were fasted and fed glucose (in an oral glucose tolerance test, OGTT), peptide 8 introduced subcutaneously performed comparably to Exendin-4. It lowered glucose levels at lower doses, and had a longer duration of action compared to Exendin-4.
Heptares was co-founded by Dr Fiona Marshall, who is currently their Chief Scientific officer. Having extensive experience in molecular pharmacology from her previous work at GSK and Millennium Pharmaceuticals, she is leading the way in GPCR research. For more on her, read an interview by Cell.
Authors of the GLP-1 cryo-EM study also involve highly esteemed GPCR researchers including Dr Brian Kobilka (Stanford), who won the Nobel Prize for Chemistry in 2012 for his work on GPCRs with Dr Robert Lefkowitz (Duke). He has founded a company called ConfometRx, which his wife Tong Sun Kobilka manages, and also focuses on GPCR-based drug development.
So it appears the GPCR field will continue to thrive. For a more detailed look into the history of GPCRs, read Dr Robert Lefkowitz’s Nobel Prize lecture.